Answer MET Question 27
Question : With reference to alkaline batteries used on board ship.
2. After a 10 hour discharge a lead-acid cell voltage will have fallen to approximately 1.73 V. The equivalent figure for an alkaline cell is 1.14 V.
3. Battery installations for both types of battery are similar in that the battery room should be well ventilated, clean and dry. Both types generate hydrogen gas during charging so smoking and naked flames must be prohibited in the vicinity of the batteries.
4. Steelwork and decks adjacent to lead-acid batteries should be covered with acid-resisting paint and alkaline resisting paint used near Ni-cd cells.
5. Acid cells must never be placed near alkaline cells otherwise rapid electrolytic corrosion to metalwork and damage to both batteries is certain For similar reasons, never use lead-acid battery maintenance gear (e.g. hydrometer, topping up bottles, etc.) on an alkaline installation or vice-versa.
6. The state of charge held by a lead-acid battery is best indicated by a test on the electrolyte specific gravity (SG) by using a hydrometer. A fully charged lead-acid cell has an SG of about 1.27-1.285 which falls to about 1.1 (or 1100) when fully discharged. The cell voltage also falls during discharge and its value can also be used as an indication of the state of charge. A lead-acid battery may be safely discharged until the cell voltage drops to approximately 1.73 V (measured while delivering load current). The state of charge of an alkaline battery cell cannot be determined from its SG value. The electrolyte density does not change during charge/discharge cycles but gradually falls during the lifetime of the battery. New alkaline cells have an SG of around 1190. When this reduces to about 1145 (which may take 5-10 years depending on the duty cycle) the electrolyte must be completely renewed or the battery replaced. Discharge of alkaline cells should be discontinued when the cell voltage has fallen to about 1.1 V.
7. The size of voltage depends on the battery type (lead-acid or alkaline) and the mode of charging, e.g.charge/discharge cycle, boost charge, trickle or float charge. Check the manufacturer's instructions for details of the required charging voltages. Do not allow electrolyte temperatures to exceed about 45 deg Cel. during charging. A lead-acid cell will gas freely when fully charged but an alkaline cell gases through out the charging period. The only indication of a fully charged alkaline cell is when its voltage remains at a steady maximum value of about 1.6-1.8 V.
8. Generally, alkaline cells are more robust, mechanically and electrically, than lead acid cells. Nickel cadmium cells will hold their charge for long periods without recharging so are ideal for standby duties. Also they operate well with a float-charge to provide a reliable emergency supply when the main power fails.
For all rechargeable batteries (other than the sealed type) it is essential to replace lost water (caused during gassing and by normal evaporation) with the addition of distilled water to the correct level above the plates. Exposure of the cell plates to air will rapidly reduce the life of the battery.
A. Describe the operation of a battery cell and state the material used;
B. Describe how the cells are mounted to form a battery;
C. State the advantages and disadvantages compared with lead-acid batteries.
Answer: A.Nickel—cadmium storage batteries Operation:
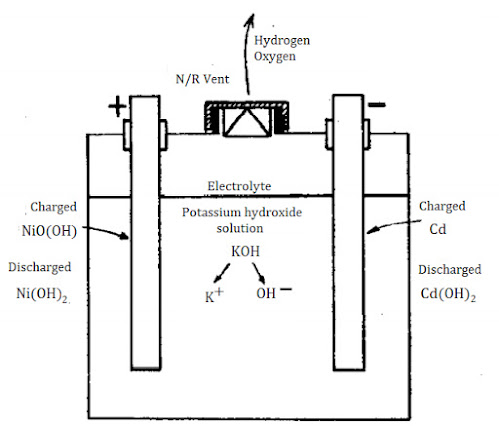
The active materials of positive and negative plates in each cell of a charged nickel-cadmium battery are nickel hydrate and cadmium, respectively. The chemicals are retained in the supporting structure of perforated metal plates and the design is such as to give maximum contact between active compounds and the electrolyte.
The active materials of positive and negative plates in each cell of a charged nickel-cadmium battery are nickel hydrate and cadmium, respectively. The chemicals are retained in the supporting structure of perforated metal plates and the design is such as to give maximum contact between active compounds and the electrolyte.
The
strong alkaline electrolyte is a solution of potassium hydroxide in
distilled water (with an addition of lithium). The ions produced in the
formation of the potassium hydroxide solution ($\displaystyle \small
\mathrm{K^+\ and\ OH^-}$ ) act as current carriers and take part in an
ion transfer.
Discharge action During discharge the complicated but uncertain action at the positive plates (hydrated oxide of nickel) causes hydroxyl ions ($\displaystyle \small \mathrm{OH^-}$ ) to be introduced into the electrolyte. As the action progresses, the nickel hydrate is changed to nickel hydroxide. Simultaneously, hydroxyl ions ($\displaystyle \small \mathrm{OH^-}$ ) from the electrolyte form cadmium hydroxide with the cadmium of the negative plates. Effectively, the hydroxyl ions ($\displaystyle \small \mathrm{OH^-}$ ) move from one set of plates to the other, leaving the electrolyte unchanged. There is no significant change in specific gravity through the discharge/charge cycle and the state of charge cannot be found by using a hydrometer.
Charging: A direct current supply for charging is obtained from a.c. mains, through the transformer and rectifier in the battery charger. The positive of the charging supply is connected to the positive of the cell, and negative to the negative terminal. Flow of current from the charging source reverses the discharge action. The reactions are complicated but can be summarised by the simplified equation:
Hydrated oxide of Nickel + Cadmium (Charged) $\displaystyle \small \mathrm{\rightarrow}$ Nickel hydroxide + Cadmium Hydroxide (discharged)
$\displaystyle \small \mathrm{2NiO(OH)+Cd+H_2O\rightarrow 2Ni(OH)_2+Cd(OH)_2}$
Gassing
The gases evolved during charging are oxygen (at the positive plates) and hydrogen (at the negative plates). Rate of production of gas increases in periods of overcharge. When hydrogen in air reaches a proportion of about 4% and up to 74% it constitutes an explosive mixture. Good ventilation of battery compartments is therefore necessary to remove gas. Equipment likely to cause sparking or arcing must not be located or introduced into battery spaces. Vent caps are non-return valves so that gas is released but contact by the electrolyte with the atmosphere is prevented. The electrolyte readily absorbs carbon dioxide from the atmosphere and deterioration results because of the formation of potassium carbonate. For this reason, cell vent caps must be kept closed.
Topping up
Gassing is a consequence of the breakdown of water in the electrolyte. This, together with a certain amount of evaporation, means that topping up with distilled water will be necessary from time to time. High consumption of distilled water would suggest overcharging.
Discharge action During discharge the complicated but uncertain action at the positive plates (hydrated oxide of nickel) causes hydroxyl ions ($\displaystyle \small \mathrm{OH^-}$ ) to be introduced into the electrolyte. As the action progresses, the nickel hydrate is changed to nickel hydroxide. Simultaneously, hydroxyl ions ($\displaystyle \small \mathrm{OH^-}$ ) from the electrolyte form cadmium hydroxide with the cadmium of the negative plates. Effectively, the hydroxyl ions ($\displaystyle \small \mathrm{OH^-}$ ) move from one set of plates to the other, leaving the electrolyte unchanged. There is no significant change in specific gravity through the discharge/charge cycle and the state of charge cannot be found by using a hydrometer.
Charging: A direct current supply for charging is obtained from a.c. mains, through the transformer and rectifier in the battery charger. The positive of the charging supply is connected to the positive of the cell, and negative to the negative terminal. Flow of current from the charging source reverses the discharge action. The reactions are complicated but can be summarised by the simplified equation:
Hydrated oxide of Nickel + Cadmium (Charged) $\displaystyle \small \mathrm{\rightarrow}$ Nickel hydroxide + Cadmium Hydroxide (discharged)
$\displaystyle \small \mathrm{2NiO(OH)+Cd+H_2O\rightarrow 2Ni(OH)_2+Cd(OH)_2}$
Gassing
The gases evolved during charging are oxygen (at the positive plates) and hydrogen (at the negative plates). Rate of production of gas increases in periods of overcharge. When hydrogen in air reaches a proportion of about 4% and up to 74% it constitutes an explosive mixture. Good ventilation of battery compartments is therefore necessary to remove gas. Equipment likely to cause sparking or arcing must not be located or introduced into battery spaces. Vent caps are non-return valves so that gas is released but contact by the electrolyte with the atmosphere is prevented. The electrolyte readily absorbs carbon dioxide from the atmosphere and deterioration results because of the formation of potassium carbonate. For this reason, cell vent caps must be kept closed.
Topping up
Gassing is a consequence of the breakdown of water in the electrolyte. This, together with a certain amount of evaporation, means that topping up with distilled water will be necessary from time to time. High consumption of distilled water would suggest overcharging.
B. Construction:
Electrolyte
Potassium hydroxide solution is strongly alkaline and the physical and chemical properties of potassium hydroxide closely resemble those of caustic soda (sodium hydroxide). It is corrosive, so care is essential when topping up batteries or handling the electrolyte. In the event of skin or eye contact, the remedy is to wash with plenty of clean water (for 15 minutes) to dilute and remove the solution quickly. Speed is vital to prevent burn damage; and water, which is the best flushing agent, must be readily available. Neutralising compounds (usually weak acids) cannot always be located easily, although they should be available in battery compartments. Specific gravity of electrolyte in a Ni—Cd cell is about 1.210 and this does not change with charge and discharge as in lead—acid cells. However, over a period of time the strength of the solution will gradually drop and renewal is necessary at about a specific gravity of 1.170.
Containers
The electrolyte slowly attacks glass and various other materials. Containers are therefore of welded sheet steel which is then nickel plated, or moulded in high-impact polystyrene. Steel casings are preferred when battieres are subject to shock and vibration. Hardwood crates are used to keep the cells separate from each other and from the support beneath. Separation is necessary because the positive plate assembly is connected to the steel casing.
Plates
The active materials for nickel—cadmium cells are improved by additions of other substances. Positive plates carry a paste made up initially of nickel hydroxide with a small percentage of other hydroxides to improve capacity and 20% graphite for better conductivity. The material is brought to the charged state by passing a current through it, which changes the nickel hydroxide to hydrated nickel oxide, NiO(OH). Performance of cadmium in the negative plates is improved by addition of 25% iron plus small quantities of nickel and graphite. Active materials may be held in pocket or sintered plates. The former are made up from nickel plated mild steel strip, shaped to form an enclosing pocket. The pockets are interlocked at their crimped edges and held in a frame. Electrolyte reaches the active materials through perforations in the pockets. Sintered plates are produced by heating (to 900'C) powdered nickel which has been mixed with a gas-forming powder and pressed into a grid or perforated plate. The process forms a plate which is 75% porous. Active materials are introduced into these voids.
Potassium hydroxide solution is strongly alkaline and the physical and chemical properties of potassium hydroxide closely resemble those of caustic soda (sodium hydroxide). It is corrosive, so care is essential when topping up batteries or handling the electrolyte. In the event of skin or eye contact, the remedy is to wash with plenty of clean water (for 15 minutes) to dilute and remove the solution quickly. Speed is vital to prevent burn damage; and water, which is the best flushing agent, must be readily available. Neutralising compounds (usually weak acids) cannot always be located easily, although they should be available in battery compartments. Specific gravity of electrolyte in a Ni—Cd cell is about 1.210 and this does not change with charge and discharge as in lead—acid cells. However, over a period of time the strength of the solution will gradually drop and renewal is necessary at about a specific gravity of 1.170.
Containers
The electrolyte slowly attacks glass and various other materials. Containers are therefore of welded sheet steel which is then nickel plated, or moulded in high-impact polystyrene. Steel casings are preferred when battieres are subject to shock and vibration. Hardwood crates are used to keep the cells separate from each other and from the support beneath. Separation is necessary because the positive plate assembly is connected to the steel casing.
Plates
The active materials for nickel—cadmium cells are improved by additions of other substances. Positive plates carry a paste made up initially of nickel hydroxide with a small percentage of other hydroxides to improve capacity and 20% graphite for better conductivity. The material is brought to the charged state by passing a current through it, which changes the nickel hydroxide to hydrated nickel oxide, NiO(OH). Performance of cadmium in the negative plates is improved by addition of 25% iron plus small quantities of nickel and graphite. Active materials may be held in pocket or sintered plates. The former are made up from nickel plated mild steel strip, shaped to form an enclosing pocket. The pockets are interlocked at their crimped edges and held in a frame. Electrolyte reaches the active materials through perforations in the pockets. Sintered plates are produced by heating (to 900'C) powdered nickel which has been mixed with a gas-forming powder and pressed into a grid or perforated plate. The process forms a plate which is 75% porous. Active materials are introduced into these voids.
C. Alkaline vs Acid batteries:
1.
The nominal cell voltages of each type are 2V for lead-acid and 1.2V
for alkaline. Hence, twelve lead-acid cells or twenty alkaline cells
must be connected in series to produce a nominal 24 V. 2. After a 10 hour discharge a lead-acid cell voltage will have fallen to approximately 1.73 V. The equivalent figure for an alkaline cell is 1.14 V.
3. Battery installations for both types of battery are similar in that the battery room should be well ventilated, clean and dry. Both types generate hydrogen gas during charging so smoking and naked flames must be prohibited in the vicinity of the batteries.
4. Steelwork and decks adjacent to lead-acid batteries should be covered with acid-resisting paint and alkaline resisting paint used near Ni-cd cells.
5. Acid cells must never be placed near alkaline cells otherwise rapid electrolytic corrosion to metalwork and damage to both batteries is certain For similar reasons, never use lead-acid battery maintenance gear (e.g. hydrometer, topping up bottles, etc.) on an alkaline installation or vice-versa.
6. The state of charge held by a lead-acid battery is best indicated by a test on the electrolyte specific gravity (SG) by using a hydrometer. A fully charged lead-acid cell has an SG of about 1.27-1.285 which falls to about 1.1 (or 1100) when fully discharged. The cell voltage also falls during discharge and its value can also be used as an indication of the state of charge. A lead-acid battery may be safely discharged until the cell voltage drops to approximately 1.73 V (measured while delivering load current). The state of charge of an alkaline battery cell cannot be determined from its SG value. The electrolyte density does not change during charge/discharge cycles but gradually falls during the lifetime of the battery. New alkaline cells have an SG of around 1190. When this reduces to about 1145 (which may take 5-10 years depending on the duty cycle) the electrolyte must be completely renewed or the battery replaced. Discharge of alkaline cells should be discontinued when the cell voltage has fallen to about 1.1 V.
7. The size of voltage depends on the battery type (lead-acid or alkaline) and the mode of charging, e.g.charge/discharge cycle, boost charge, trickle or float charge. Check the manufacturer's instructions for details of the required charging voltages. Do not allow electrolyte temperatures to exceed about 45 deg Cel. during charging. A lead-acid cell will gas freely when fully charged but an alkaline cell gases through out the charging period. The only indication of a fully charged alkaline cell is when its voltage remains at a steady maximum value of about 1.6-1.8 V.
8. Generally, alkaline cells are more robust, mechanically and electrically, than lead acid cells. Nickel cadmium cells will hold their charge for long periods without recharging so are ideal for standby duties. Also they operate well with a float-charge to provide a reliable emergency supply when the main power fails.
For all rechargeable batteries (other than the sealed type) it is essential to replace lost water (caused during gassing and by normal evaporation) with the addition of distilled water to the correct level above the plates. Exposure of the cell plates to air will rapidly reduce the life of the battery.

Comments
Post a Comment